VECTOR COMPETENCE OF Ixodes holocyclus
About biological vectors
Ixodes holocyclus as a vector - Spotted Fevers, Q Fever, Lyme-like
Disease, Viral Diseases
Article: Lyme Disease in Australia? (by Richard C Russell, Westmead Hospital, 1995)
About biological vectors
In general terms a vector may simply mechanically transport a pathogen without it replicating, or it may both transmit and replicate a pathogen. Many viruses, bacteria, rickettsia and protozoal organisms are capable of replicating within ticks. This is known as "biological" transmission and this mode of transmission is the one that applies most commonly to ticks. For hard (ixodid) ticks it is virtually impossible for mechanical transmission to occur on its own (i.e. without some replication), as they tend to not take multiple blood meals from different hosts in the one stage. Trans-stadial transmission, it seems, requires that an organism can replicate within the host.
Ixodes holocyclus as a vector
In the case of Ixodes holocyclus there is much speculation about transmission of various pathogens but so far little in the way of hard facts, with the exception perhaps of Queensland Tick Typhus (Rickettsial Spotted Fever). Saying that an arthropod is a competent vector (generally) requires several findings: (1) frequent isolation of the suspected pathogen; (2) showing that the distribution of the suspected vector overlaps with a diseased population (be it human or animal); (3) showing that host-seeking and blood-feeding coincide with the disease incidence; (4) showing that disease incidence varies with host choice; and (5) showing the capacity for vector competence in the laboratory.
Spotted Fevers
For example with respect to the paralysis tick I. holocyclus (and I. tasmani) and the transmission of the pathogen R. australis (Rickettsial Spotted Fever), only points 2,3, and 4 are valid. Regarding point 1, isolations have been reported from only the one paper (Campbell and Domrow, 1974), although the rickettsia were isolated on three occasions. These days there is not much disputing that I. holocyclus is the main vector for R. australis, unfortunately vector competence work is still required to confirm its status. (S. Doggett pers. com.)
Q Fever
Despite the lack of vector competence studies, the paralysis tick (Ixodes holocyclus) is commonly also mentioned as a potential vector of Q-fever (caused by the rickettsial organism Coxiella burnetii). The ornate kangaroo/wallaby ticks Amblyomma triguttatum s.l. have also been implicated in Q-fever (Russell and Doggett, 1995).
Lyme-like Disease
Some vector competence studies have been undertaken on I. holocyclus with respect to the Lyme disease pathogen Borrelia burgdorferi sensu stricto (with a United States strain). These suggested that the tick can NOT transmit this strain of spirochaete (Piesman and Stone, 1991). Despite this, there is a strong belief that some kind of Lyme-like spirochaete causes a Lyme-like disease in Australia and that it is carried by the most common tick afflicting humans, Ixodes holocyclus. At the Royal North Shore Hospital in Sydney the Tick-borne Diseases Research Unit continues its study into this issue. Cases of Lyme-like disease are being diagnosed on the basis of clinical signs (often musculoskeletal, chronic fatigue, neurological and dermatological), exclusion of other infections, serology (which supportive but not conclusive), and response to antibiotic treatment (initially, antibiotics may cause a worsening of symptoms, presumably as spirochaetes are destroyed, and this in part supports the diagnosis). In Sydneys northern suburbs those people so diagnosed are colloquially known as "Lymies". The Australian form of Lyme-like borreliosis is associated with a different clinical picture from true Lyme disease of North America. For more detailed discussion see information provided by TAGS (Tick Alert Group Support). See also the Westmead Hospital Dept of Medical Entomology site for their interpretation on the status of an Australian Lyme-like Disease. An article by Richard Russell is copied below- it gives descriptions of some of the clinical syndromes seen in Australia.
Viral Diseases
So far, no viruses have been isolated from I. holocyclus (Russell and Doggett, 1995).
See also tick-transmitted diseases in dogs and man.
Article:
by Richard C. Russell Department of Medical Entomology, Centre for Infectious Diseases and Microbiology, University of Sydney and Westmead Hospital, Westmead, Australia
in EMERGING INFECTIOUS DISEASES Volume 1, Number 1, January-March 1995 Dispatches
Lyme Disease in Australia?-Still To Be Proven!
To the Editor: The first case of a syndrome consistent with Lyme disease was reported from the Hunter Valley region of New South Wales (NSW) in southeastern Australia in 1982, but there was no confirming serology. More clinical cases, again without serologic confirmation, were reported in 1986, two from the south coast and one from the central coast of NSW. The Queensland State Health Laboratories reported that 186 (14.9%) of 1,247 sera taken from patients between 1986-1989 showed antibody response to Borrelia burgdorferi of ò64 by indirect fluorescence antibody test (IFAT), but none of these results were confirmed by immunoblotting. In 1988, a multidisciplinary investigation of putative Lyme disease began, encompassing clinical, serologic, vector, and reservoir host studies, and results from these studies have been published (1). What follows herein is derived from the accumulated published and unpublished data of the research team, the members of which are credited in the acknowledgments.
Over the past 6 years, principally because of local publicity, there has been an increase in serologic testing for Lyme disease in Australia, particularly in southeastern Australia. Testing has often been initiated by patients with undiagnosed health problems. Thus, most Lyme disease patients seen by infectious disease specialists are self-selected and are referred for assessment on the basis of tick exposure and reported positive serologic test results for Lyme disease. Patients with positive serologic test results frequently have long-standing symptoms for which no other diagnosis has been established.
The most common symptoms are musculoskeletal, including myalgias and arthralgias without objective evidence of joint swelling, and syndromes involving fatigue and loss of energy resembling chronic fatigue syndrome. Some patients fulfill diagnostic criteria for fibromyalgia. The next most common symptoms are neurological, and include frequent headaches, inability to concentrate, and memory loss. The most common dermatologic manifestation of chronic Lyme disease, acrodermatitis chronica atrophicans, seen occasionally in Europe and rarely in the United States, has not been reported from Australia. A few cases of erythema migrans, the characteristic dermatologic manifestation of acute Lyme disease, have been reported from southeastern Australia, but clinical diagnosis can be confounded by hypersensitivity reactions to tick bite; a spectacular erythematous reaction is often associated with the bite of Ixodes holocyclus, the most common tick biting humans in NSW.
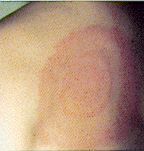 |
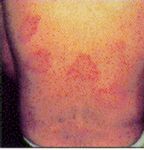 |
These examples of "erythema migrans" (EM) rashes have been inserted into this article by the web author; note that considerable variation in appearance and location occurs; source: the Texas Department of Health. |
Only eight specimens submitted to our laboratory included skin biopsies done to isolate spirochetes. B. burgdorferi s.l. was isolated from one patient returning from Europe, but no spirochetes were isolated from local patients. In our serologic diagnostic service, an enzyme-linked immunosorbent assay (ELISA) for IgG and an IFAT for IgG and IgM have been used with antigens derived from North American B. burgdorferi strain B31 (2). From 1988 to April 1994, 78 (1.8%) of 4,372 local patients were positive for IgG by both methods. All 78 patients were tested by IgG Western blot for confirmation by using the virulent North American B. burgdorferi strain 297 and a German strain designated B7: with B. burgdorferi strain 297, 46 patient samples showed as many as four indicative bands; with the European strain B7, 22 patient samples showed as many as three indicative bands; bands used were 18, 21, 28, 30, 31, 34, 39, 41, 45, 58, 66, 83, and 93 kDa, modified from Dressler et al (3). Twenty-four other patients with various bacterial, viral, or autoimmune syndromes not relating to Lyme disease were tested as controls: with strain 297, 11 control samples showed as many as two indicative bands, and with strain B7, 10 control samples showed as many as two indicative bands. A high degree of cross-reactivity was demonstrated with the controls, particularly with respect to the 31, 41, 58, and 66 Kda bands for both the European and the American antigen. As none of the 78 patients, including putative late-stage patients positive by ELISA and IFAT, showed more than four specific bands to either antigen, they would be considered negative by the criteria of Dressler et al (3). Fewer than 1% of all referred patients conformed with the national surveillance case definition used in the United States by the Centers for Disease Control and Prevention.
Problems of specificity and sensitivity associated with serologic testing for Lyme disease are well recognized, particularly in Australia where no local spirochete has been isolated for use as a reference antigen. Seroprevalence rates for B. burgdorferi infection in humans have been compared between 200 high (rural residents) and 200 low (urban residents) tick exposure groups in coastal NSW, by using the IgG ELISA. No significant difference was found between the two groups, and the overall seropositivity rate was 2.2% (9/400). A parallel survey of dogs in NSW has shown a similar result with an overall seropositivity rate of 2.5% (6/239). These results contrast with those reported from known endemic-disease areas outside Australia that have rural populations with considerably higher seropositive rates. The low rate found by our surveys is similar to that found by other studies undertaken in areas where Lyme disease is not endemic, and humans have 1%-3% positive serologic results cause by cross-reacting antibodies (4). From January 1990 to December 1992, ticks were collected in areas associated with putative Lyme disease cases and were examined for spirochetes to detect a possible causative agent in potential vectors. Ticks were collected along the east coast of Australia, from southern Queensland through NSW into northern Victoria, by flagging in natural habitats, and from domestic and other native animals. Detection of spirochetes was attempted by dark-field microscopy and culturing of gut contents and by direct testing of ticks using polymerase chain reaction (PCR) to detect the Borrelia-specific flagellin gene (5). In total, more than 12,000 (>1,000 by PCR) ticks were processed, including 7,922 I. holocyclus (1). No spirochetes were detected by dark-field microscopy or by PCR. Spirochete-like objects (SLOs), were observed in 94 cultures from bloodfed ticks and only in cultures with bacterial contaminants, presumably from the bloodmeal. Some SLOs yielded positive fluorescence results when tested with Borrelia-specific polyclonal antibodies, but tests with monoclonal antibodies (anti-flagellin H9724, anti-OspA h3332, anti-OspB h4831) were negative. Electron micrographs showed that the SLOs were not typical of Borrelia, were composed of fibers, and probably were not spirochetes. The electron micrographs were similar to micrographs of SLOs recovered from contaminated cultures from ticks in the United States and Europe and thought to be composed of aggregations of bacterial flagella, probably from the contaminants. Molecular characterization indicated that the SLOs were not related to B. burgdorferi. A small number of native vertebrate animals (13 native rats Rattus fuscipes, 3 bandicoots Perameles nasuta, and 1 marsupial mouse Antechinus stuartii) trapped on the south coast of NSW were sampled by ear-punch biopsy (6) for culture and PCR investigation, but no evidence of borreliae was found. The animal sample was clearly inadequate, and the PCR primers used for the tick and animal studies may have been inappropriate and unable to identify native Australian spirochetes; however, the extensive investigations of tick gut contents by culturing and dark field microscopy were also negative for borreliae. There are some major differences between Australia and the Lyme-disease-endemic areas of the Northern Hemisphere with respect to the natural history of borreliosis. No ticks of the I. persulcatus complex, the principal vectors to humans in the northern hemisphere, occur in Australia.
In eastern Australia, the logical candidate vector would be I. holocyclus, which has a wide host range and is the most common tick biting humans. I. holocyclus cannot transmit a North American strain of B. burgdorferi (7) but the association with any possible Australian spirochetes remains unresolved. Likewise, none of the mammal species identified as reservoir hosts in the Northern Hemisphere are present in Australia. There are reports of spirochetes in Australian native animals, and a local mammal could be a reservoir host for an indigenous spirochete that occasionally infects humans through a tick vector and produces a clinical syndrome similar to Lyme disease; however, no spirochete was detected in the ticks or animals studied. The diagnosis of Lyme disease outside known disease-endemic areas should not be based solely on serology because unrelated syndromes, such as autoimmune diseases and cross reactions with other bacteria, can produce false-positive results. Likewise, a definitive diagnosis on clinical grounds alone in a nonendemic-disease area is difficult to justify without adequate scientific support based on isolation of the causative agent from the patient or from another patient or known vector from the region. In Australia, disagreement as to what constitutes a positive serologic result has additionally contributed to overdiagnosis of Lyme disease. Until an organism is isolated from a local patient and is characterized, the presence of Lyme disease in Australia will remain controversial.
Acknowledgments
My colleagues Rosemary Munro (clinical microbiology), Department of Microbiology, Liverpool Hospital, and Stephen Doggett (entomology), David Dickeson (serology), Danielle Avery (molecular biology), Cheryl Hunt (molecular biology), Joanne Mercer (microbiology), and Nicole Trivett (electron microscopy), CIDM at Westmead Hospital, and John Ellis (molecular biology), Department of Microbiology, University of Technology Sydney, contributed to the studies outlined in this paper. Richard Lawrence, Clinical Superintendent of Medicine, Westmead Hospital, provided valuable discussions on clinical aspects and case presentation. Our investigations were supported by the National Health and Medical Research Council and the Ramaciotti Foundations.
Richard C. Russell Department of Medical Entomology, Centre for Infectious Diseases and Microbiology, University of Sydney and Westmead Hospital, Westmead, Australia
References
1. Russell RC, Doggett SL, Munro R, Ellis J, Avery D, Hunt C,
Dickeson D. Lyme disease: a search for the causative agent in
ticks in southeastern Australia. Epidemiol Infect 1994;112:375-84.
2. Russell H, Sampson JS, Schmid GP, Wilkinson HW, Plikaytis B.
Enzyme-linked immunosorbent assay and direct immunofluorescence
assay for Lyme disease. J Infect Dis 1984;149:465-70.
3. Dressler F, Whalen JA, Reinhardt BN, Steere AC. Western
blotting in the serodiagnosis of Lyme disease. J Infect Dis 1993;167:392-400.
4. Barbour AG, Fish D. The biological and social phenomenon of
Lyme disease. Science 1993;260:1610-6.
5. Persing DH, Telford III SR, Rys PN, Dodge DE, White TJ,
Malawista SE, Spielman A. Detection of Borrelia burgdorferi DNA
in museum specimens of Ixodes dammini ticks. Science 1990;249:1420-3.
6. Sinsky RJ, Piesman J. Ear punch biopsy method for detection
and isolation of Borrelia burgdorferi from rodents. J Clin
Microbiol 1989;27:1723-7.
7. Piesman J, Stone BF. Vector competence of the Australian
paralysis tick, Ixodes holocyclus, for the Lyme disease
spirochaete Borrelia burgdorferi. Int J Parasitol 1991;21:109-11.
E-mail Us to report a broken link!